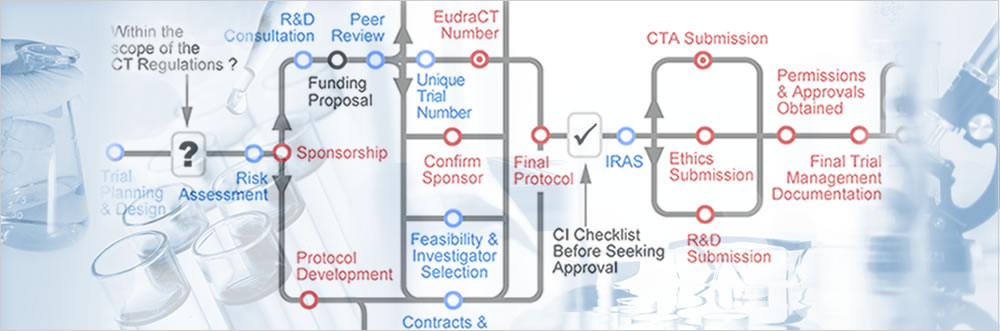
The Clinical Trials Toolkit
The Clinical Trials Toolkit provides practical advice to researchers in designing and conducting publicly funded clinical trials in the UK. Through the use of an interactive routemap, this site provides information on best practice and outlines the current legal and practical requirements for conducting clinical trials.
The Toolkit is primarily focused on Clinical Trials of Investigational Medicinal Products (CTIMPs) and the regulatory environment and requirements associated with these. However researchers and R&D staff working on trials in other areas will also find useful information and guidance of relevance to the wider trials environment.
Latest Clinical Trials Toolkit news

How to be a good Chief Investigator for clinical trials - New Date
A one day workshop is being held in Cardiff in November 2024, on 'How to be a good Chief Investigator for clinical trials'
Trial Steering Committee Workshop: A Primer for TSC Members
A online Trial Steering Committee workshop is being held on 06 June 2024, organised by Health Research Board – Trial Methodology Research Network & Irish Critical Care Clinical Trials Network in Ireland and the Trials Methodology Research Partnership in the UK
Clarification of HRA policy on the registration of CTIMPS in the UK and EU
CTIMPs taking place in the UK as well as in countries in the EU, must be registered with both the CTIS and a WHO primary registry or ICMJE approved registry
